Article by Norm Brandes and Keith McCord
Ethanol, or moonshine whiskey has been used as either fuel or liquor since the mid-15th century. The very first patented internal combustion engine was invented by Samuel Morey back in 1826 and ran on a mixture of ethanol, alcohol and turpentine. Fast forward to 1861 where German inventor Nicolaus Otto, also known as the father of the modern four stroke engine, developed an internal combustion engine that ran on pure ethyl alcohol with a heated carburetor. Why? Lamp alcohol at the time was untaxed by the German government and, frankly, gasoline really wasn’t available.
Jump ahead to the dawn of the automotive age and meet the first flex fuel vehicle. Henry Ford introduced the Model T to run on both ethanol and gasoline. Since there weren’t really any gasoline service stations back in the early 20th century, and distilling moonshine was everywhere, it only made sense from a demand/supply standpoint. In effect, the internal combustion engine was really designed and developed around alcohol and not gasoline.
Hooch: It’s Not Just For Drinkin’ Anymore
Gasoline, ethanol, methanol, propane, butane, and kerosene are all included in the family of hydrocarbons. Simply stated, a hydrocarbon is a chemical substance built exclusively of hydrogen and carbon atoms. A good rule of thumb is if it is a fuel and it burns, odds are it is in the hydrocarbon family.
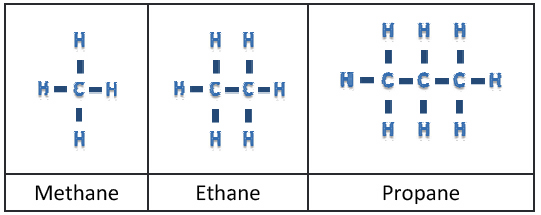
Simple hydrocarbons such as methane, ethane, propane and octane are shown in (Figure 1) and are found in gaseous form. As we add more carbon chains, we start to get liquid simple hydrocarbons.
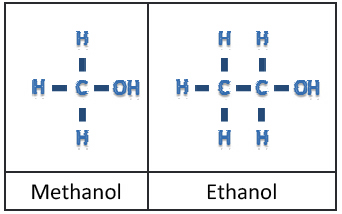
As we continue to create longer chains, we go from gases, to liquids, to waxy solids. The next step is to bond an oxygen atom to one of the hydrogen atoms to for a hydroxyl group. This takes the simple hydrocarbon and makes what we call simple alcohols shown in (Figure 2). You can continue to create these chains of atoms to achieve more and more complex alcohols.
I’ll Take My Whiskey Neat Please
Any engine builder worth his or her salt knows that when you design a race engine to run on alcohol, you always have to take into account the requirement of the extra fuel required. This may be as simple as changing out carburetor jets, upping the size of fuel injectors, or even redesigning the fuel delivery system to larger lines, multiple pumps, etc. These rules aren’t folklore created by fuel salesmen and the aftermarket; these rules all have a scientific reason behind them.
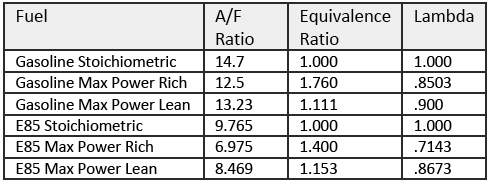
If you study the diagrams for both Methanol and Ethanol, and dust off your old high school periodical chart, we can break down the atomic weights as follows: Hydrogen = 1, Carbon = 12, Oxygen = 16.
Thus, a methanol molecule’s atomic weight is 32 (4 hydrogens, 1 carbon, 1 oxygen) while ethanol’s atomic weight is 46 (6 hydrogens, 2 carbons, 1 oxygen). What happens during combustion is an interesting process. The OH atoms that were added to the base hydrocarbon to create the alcohol break off and team with another hydrogen atom. This combination makes H2O, or what we know as water.
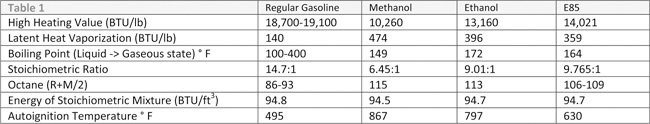
So unlike gasoline, a byproduct of alcohol combustion is water. Given that the atomic weight of water is 18, you can see that in the case of ethanol, 39 percent of the molecule cannot be burned and does not contribute to power added. Gasoline has a heat value of 20,400 BTUs while ethanol has a heat value of 12,800 BTUs. This means when compared to gasoline, ethanol will have only 60 percent of the heat value of gasoline.
Why would you want to burn a fuel that has 60 percent of the available heat of gasoline and has incombustible water as a product of combustion? The secret lies in the Latent Heat Vaporization, Autoignition Temperature and Energy of Stoichiometric Mixture of alcohols.
All Things Being Equal
The first striking attribute in Table 1 is that the Energy of Stoichiometric Mixture is roughly the same. What this essentially means is that when the engine is setup to run at the given Stoichiometric mixture, a cubic foot of any of the four fuels will yield roughly the equivalent energy (94.8 vs 94.5 vs 94.7). Thus, the volumetric efficiency of each of the fuels on a properly designed engine will be the same. Therefore, once the carburetor or fuel injection system is set up to give the proper air/fuel ratio for the fuel, the engine should be ready to run.
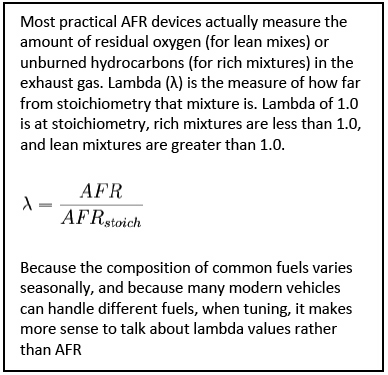
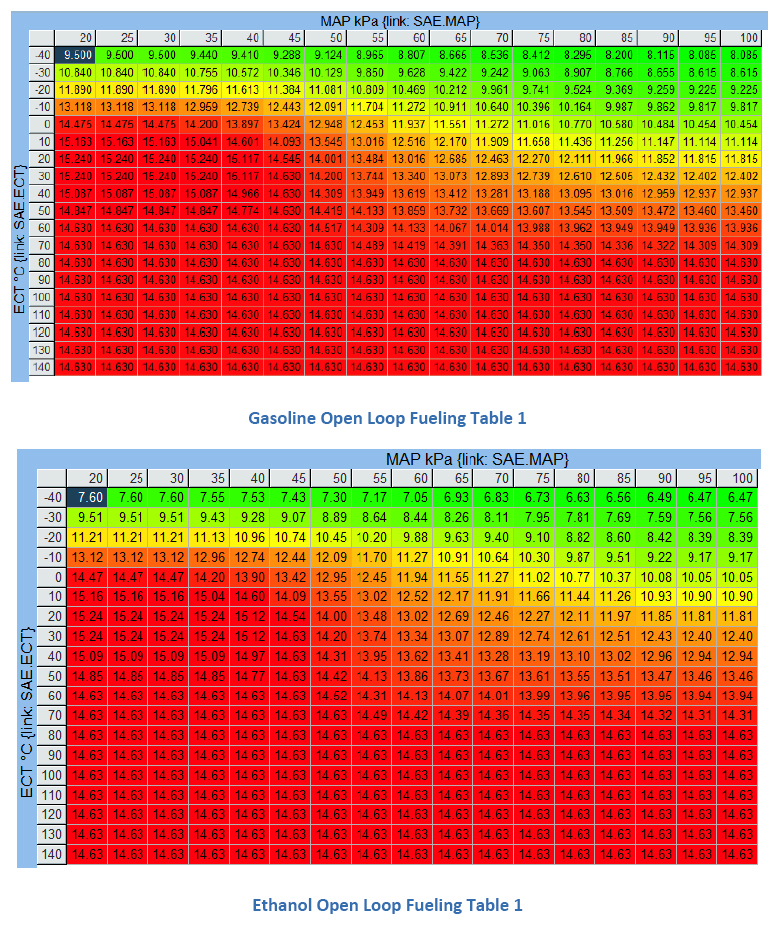
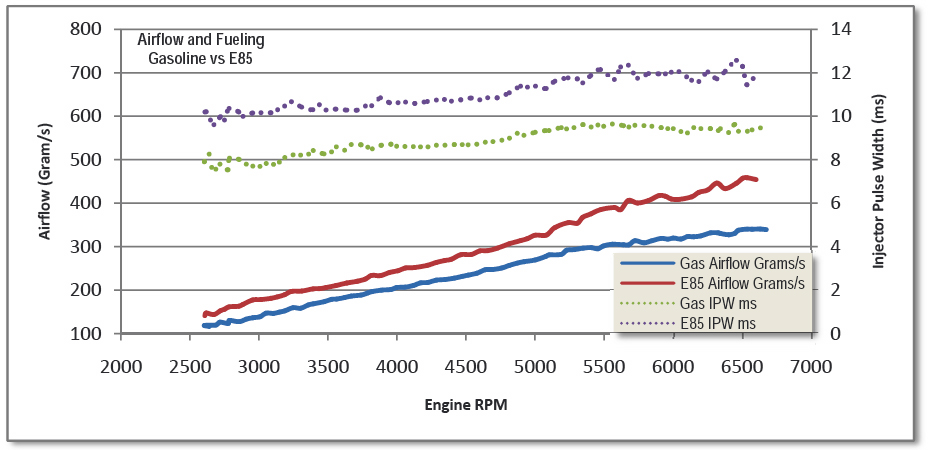
A peek inside a GM flex-fuel ECM reveals that there’s only one volumetric efficiency table regardless of the fuel used. We’ve already proven that our energy at stoich is equivalent. Looking at the open loop fueling tables we notice that alcohol requires more fueling with colder temperatures. However, as engine temperatures increase, the same air fuel ratio is commanded for both gasoline and E85. What allows this to occur is both fuels have a lambda value of 1.000 at stoich. Therefore, we can use both narrow band and wide band oxygen sensors to monitor and trim fuel. Remaining focused on the lambda values and not the readings on the air/fuel ratio meter is the first step to success.
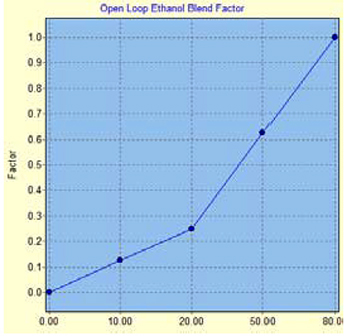
GM measures the percentage of ethanol in flex-fuel with a sensor in the fuel delivery system. Based on this sensor’s output, it will balance the fueling between the two tables. As more ethanol is sensed, the open loop fueling will be biased more towards the ethanol fueling table up to the point where we are running unmixed E85 only from the ethanol table.
Boil It To Cool It Down
If you’ve ever poked around an alcohol-fueled engine equipped with carburetors, you probably noticed that the intake runners are iced over during idle, especially if equipped with a large overlap camshaft. You probably also noticed that the engine runs quite a bit cooler when running alcohol. What causes this? Well, you’ve probably experienced this phenomenon before. Put a bit of water on exposed skin, then gasoline and then alcohol. The alcohol, as it evaporates, will feel the coolest, followed by gasoline, then water. This is all thanks to the Latent Heat Vaporization property of liquids.
Physics dictates that a certain amount of heat is required to convert a liquid to gas. This additional heat is expressed as the Latent Heat Vaporization value. Ethanol requires about 250 percent of the heat to change to a gas, and methanol requires about 340 of the heat. So, in effect, the conversion from liquid to gaseous state will draw heat from the combustion chamber. We know that gasoline will drop the chamber temperature about 40° F, therefore, ethanol will drop the temperature roughly 100° F while methanol will drop it roughly 135° F. Thus, at these Stoichiometric values, we see a significant drop in temperature of the air/fuel mixture. This is one of the reasons that we like to use supplemental alcohol injection on forced induction engines even if they are running gasoline.
The Ideal Gas Law states that as the temperature of the air/fuel mixture drops, our mass density of the charge increases. Furthermore, the volumetric mass efficiency will also increase, resulting in more power as our engine’s volumetric efficiency values grow.
Timing (And Compression) Is Everything
We now have more alcohol to burn and a cooler air charge, but what about lighting the charge? One key factor to refer back to our table is the Autoignition for each fuel. The alcohols have significantly higher Autoignition temperatures as compared to gasoline. This enables you to design an engine with higher static and dynamic compression ratios. As you know, this is an easy way to make more horsepower and torque in an engine. Since we have much higher Autoignition temperatures versus gasoline, we don’t have to worry as much about pre-ignition with the raised compression ratios.
Also, take note of the octane numbers. Alcohols range from a low of 106 octane to a high of 115 octane. As a result, we have a much more knock-friendly fuel as compared to gasoline. This allows us to design a lot more timing into the engine than we could have with gasoline. With the higher octane numbers, we don’t have to be as worried about detonation with the higher timing values. Another problem we ran into in the past was increased timing usually meant that the engine ran hotter and we’d have to design around this issue to cool the engine. But, we already know with our Latent Heat Vaporization values of alcohol, we are removing a significant amount of heat from the chamber, so in effect, an alcohol motor is not temperature affected by timing increases.
Finally, with the increase in compression ratio, we also get more heat in the chamber, which equates to not only a better burn, but also better vaporization.
One example is how GM handled its flex-fuel timing issues by creating an “adder” table where they either added or subtracted timing from the base gasoline timing tables. As the load and rpm increase, timing also increases reaching its highest level around peak torque.
It Isn’t a Panacea
Under lower temperatures, the alcohol in the air/fuel mixture will revert back to its liquid state and fall out of suspension in the mixture. In the liquid state, the alcohol is very difficult to ignite and start the combustion process. Enter E85, which is typically what is available at gasoline stations across the country. E85 is typically a blend of 85 percent ethanol, 15 percent gasoline, although during winter months in cold climates, it may slip to a 70/30 blend.
The addition of gasoline makes the cold starts easier so that heat may get into the engine and get up to temperature easily. You should remind your customer of the 70/30 winter blend possibility and set the engine up appropriately.
Another key factor that will cause E85 issues is water. The introduction of water, in concentrations as little as one percent, will cause the gasoline and ethanol to separate. Most new vehicles that employ closed fuel systems will not have any problems. But, on open fueling systems, or in instances where water has been introduced to the fuel system, adding benzene, acetone and butyl alcohol will increase the water tolerance of E85.
Emissions Considerations
Why not use race gas? Well, first thing, leaded race gas will knock out an oxygen sensor rather quickly. Secondly, from an environmental standpoint, it isn’t very friendly. As far as unleaded race gas goes, there isn’t much available above 104 octane.
Alcohol-fueled engines emit carbon dioxide (CO2)and water (H2O). Both of these byproducts are extremely environmentally friendly and non-poisonous. Unfortunately, it is next to impossible to achieve a perfect burn, so as a result, carbon monoxide (CO) is also present, which is poisonous and is watched carefully by the EPA. But, an alcohol-based fuel will result in significantly reduced CO emissions when compared to a gasoline-fueled engine.
According to EPA studies, when using ethanol over gasoline:
• Carbon monoxide (CO) reduced by 25-30%
• Nitrogen oxides (NOx) reduced by up to 20%
• Emissions of Volatile Organic Compounds (VOCs) reduced by 30%
• Emissions of cancer-causing benzene and butadiene reduced by
more than 50%
• Significantly reduced Sulphur dioxide (SO2) and Particulate
Matter (PM)
Tests by the EPA have shown negligible differences in the above percentages when comparing ethanol (E100) and a blended ethanol/gasoline mixture (E85).
Ethanol is also emissions sensor friendly. Standard heated exhaust gas oxygen sensors will work in their narrow or wide band modes to verify Stoichiometric values of the spent gasses in the exhaust pipes. Therefore, when tuning an ethanol-based vehicle, no additional sensors are required either in the car or on the dynamometer.
Adapting To E85
Our first case study is a newly freshened GM Gen III engine. Aftermarket cylinder heads, camshaft, and a forged lower end were assembled with the idea of digesting roughly 200-250 hp of nitrous oxide. The problem was that while on the motor only, pump gas was not an issue, while on nitrous, a separate fuel cell dedicated to the nitrous system, and filled with C16 race gas was required. The idea was to convert over to alcohol to eliminate this costly additional fuel cell. Since this engine was designed to run on pump gas, the static compression was kept fairly low for a Gen III engine at 11.3:1. The camshaft was set up for nitrous usage, so it bleeds off a lot of compression, yielding a very low dynamic compression value of 8.2:1.
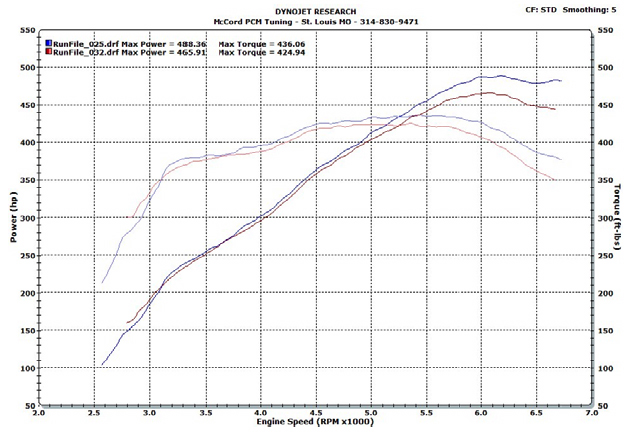
This motor wasn’t a good candidate for running E85 on motor only, but, with the size of the nitrous shot, we forged ahead. To keep a fair comparison, we swapped to a 60 lb.hr injector set and strapped the car to a Dynojet dyno and began tuning. First, we did E85, then drained the tank and ran 92 octane pump gas.
Our back-to-back test (after tuning) showed an increase in both horsepower and torque, although the increases weren’t significant. The lack of increase was mainly due to the low static and dynamic compression ratios.
Engine Design With Alcohol In Mind
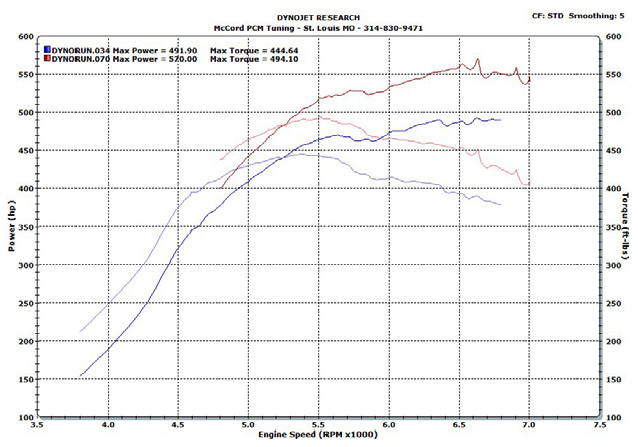
When designing an engine from the ground up knowing that it will run on alcohol, you can take advantage of compression and temperature. In this study, a 532 cid big block Chevrolet engine was converted to fuel injection and set up to run purely on alcohol (methanol or ethanol). Static compression was set up at 12.5:1 with dynamic compression at 9.3:1. Due to the higher compression ratio, we were unable to run pump gas for our test, but we did use 106 octane leaded race gas. We were able to make significantly more power with a a properly designed and tuned E85 engine!
About the Authors: Norm Brandes owns and operates Westech Automotive, Inc., a machine shop and vehicle repair service business located in Silver Lake, WI. He is as wizard in gaining performance while keeping vehicles within emissions standards. Keith McCord is president of McCord Consulting Group in St. Louis, MO. He’s a “gun-for-hire” for various OEM and aftermarket companies to design products, parts, CNC code and process improvement. Keith also writes custom ECMs for GM and Ford vehicles.