Cleaning is being done every day and is an integral part of many businesses and their processes. One of the most commonly asked questions about using sodium bicarbonate (a.k.a. baking soda) as a blasting abrasive is simply: How does it work?
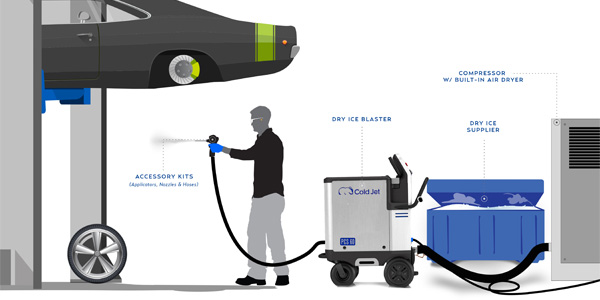
Let’s start with: What is abrasive blasting? Abrasive blasting uses either pressurized air or water containing a suspended particulate (the abrasive media) that is projected at a surface for the purpose of removing a contaminant. How does baking soda blasting work? Baking soda blasting can be done wet or dry. Both methods require compressed air or water to deliver the baking soda. In addition, baking soda is a soft crystal (Moh’s hardness of 2.5). It is harder than the contaminants you are looking to remove, be it grease, oil, or paint but softer than the substrate. This is how it’s able to clean without damaging the underlying surface. By comparison, when a hard-abrasive particle impacts the surface, the energy of the impact is transferred into the substrate, fracturing it and blowing materials off. When baking soda impacts a substrate, the impact energy is transferred back into the baking soda crystal itself, which gets fractured and blown apart. The substrate remains unchanged.
Baking soda is the perfect abrasive blasting media for the removal of oil and grease, and this is why: Grease and oil, both petrochemically and agriculturally sourced, dissolve in an alkaline environment. Baking soda has a pH of 8.25, making it a mild alkaline material. During soda blasting, an alkaline environment is created by the distribution of baking soda particles all over the substrate (the part) being cleaned. These particles absorb the oil and grease like a sponge because the oil and grease favor the alkaline environment over the substrate. The result is a clean and oil and grease free surface, all in one-step.
Picture a greasy cylinder head or crankcase and imagine how much ‘elbow grease’ you would have to use to remove all the oil and grease. The traditional way to clean parts is to pre-treat it in a chemical solvent bath and then treat it a second time in a solvent bath than hand scrubbing the part. Even after all that effort, the part you are working on might not be completely clean. It is much easier to clean parts with baking soda. As discussed, baking soda’s alkalinity naturally attracts oil and grease, thus removing the grime at the onset and saving a whole step in the process.
When multiple parts need cleaning, it may make sense to consider a cleaning technique that uses baking soda. Baking soda is a non-hazardous and nontoxic method for removing contamination all while preserving what you are cleaning. In addition, baking soda doesn’t produce sparks, and can be used to clean, degrease and remove burnt on carbon, gasket material, grime and paint on a wide variety of materials including steel, aluminum, lead, alloys, plastics, rubber and composites.
This article was sponsored by ARMEX. To learn more about how to use baking soda visit www.armex.com.